| Browse | Text search | Protein search | Domain search | Download | User manual | Related links | Citation & Contact |
The Outer membrane protein G (OmpG) Family [Function: Non-specific diffusion channels] Seed alignment | Full alignment | Pfam page | Pfam Wiki page | TC-DB page | ||||
OmpG is a monomeric porin possessing a relatively large channel approximately 2.5 nm in diameter. OmpG acts as an efficient non-specific channel for mono-, di- and trisaccharides. OmpG forms a robust 14-stranded ß-barrel with a wide, nonselective pore, which exhibits voltage- and pH-dependent gating caused by loop-driven transitions. | ||||
Representative image: 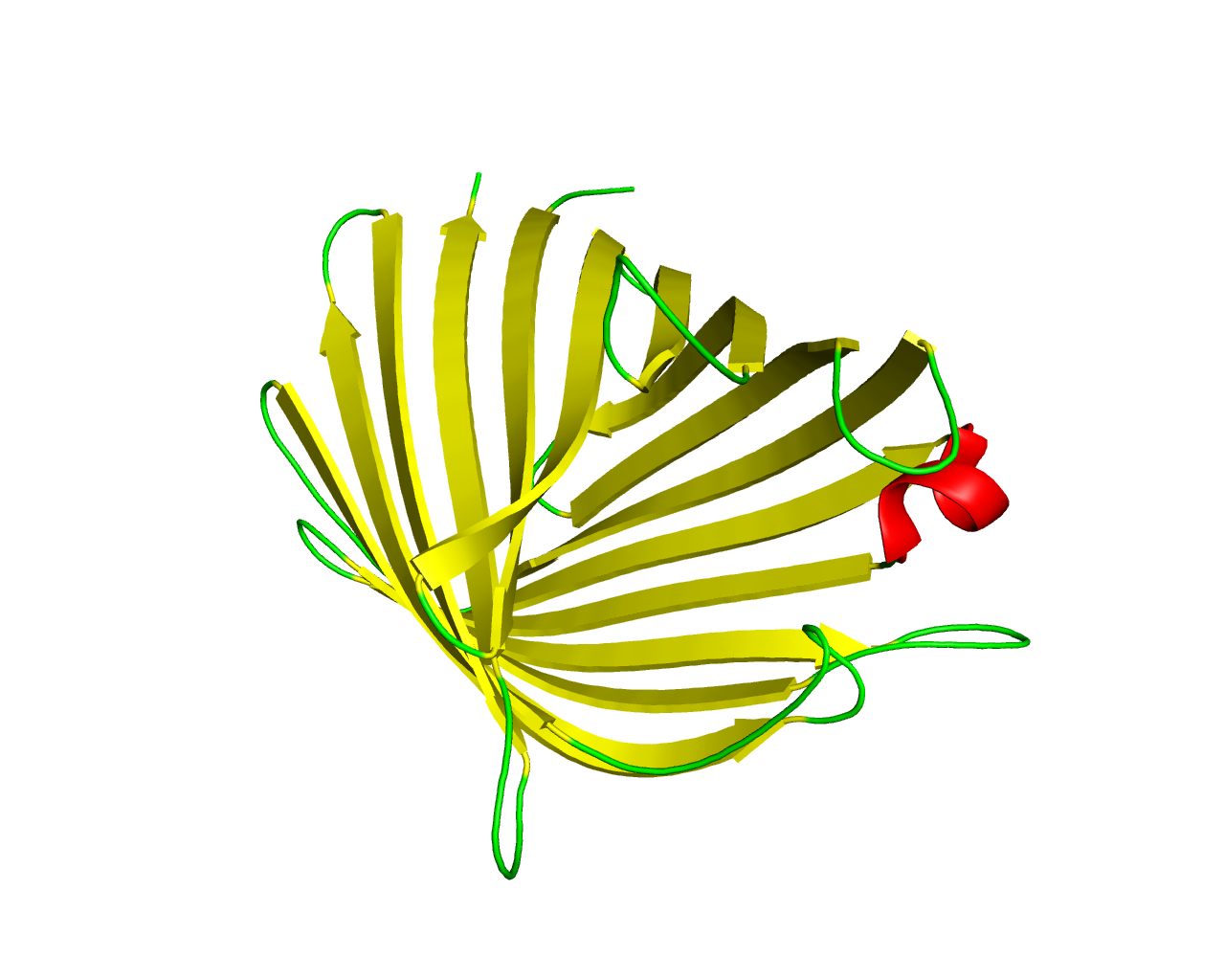 | ||||
| Literature references | ||||
Quiet Outer Membrane Protein G (OmpG) Nanopore for Biosensing ACS Sens. 2019 May 24;4(5):1230-1235. doi: 10.1021/acssensors.8b01645. Epub 2019 Apr 25. PMID: 30990011 | ||||
A light-triggered transmembrane porin Chem Commun (Camb). 2018 Aug 23;54(69):9623-9626. doi: 10.1039/c8cc05221b. PMID: 30095845 | ||||
The Bam complex catalyzes efficient insertion of bacterial outer membrane proteins into membrane vesicles of variable lipid composition J Biol Chem. 2018 Feb 23;293(8):2959-2973. doi: 10.1074/jbc.RA117.000349. Epub 2018 Jan 8. PMID: 29311257 | ||||
Engineering a Novel Porin OmpGF Via Strand Replacement from Computational Analysis of Sequence Motif Biochim Biophys Acta Biomembr. 2017 Jul;1859(7):1180-1189. doi: 10.1016/j.bbamem.2017.03.012. Epub 2017 Mar 21. PMID: 28341438 | ||||
Selective Detection of Protein Homologues in Serum Using an OmpG Nanopore Anal Chem. 2015 Nov 3;87(21):11143-9. doi: 10.1021/acs.analchem.5b03350. Epub 2015 Oct 23. PMID: 26451707 | ||||
IR-spectroscopic characterization of an elongated OmpG mutant Arch Biochem Biophys. 2015 Jun 15;576:73-9. doi: 10.1016/j.abb.2015.04.010. Epub 2015 May 6. PMID: 25958106 | ||||
Purification, Refolding, and Crystallization of the Outer Membrane Protein OmpG from Escherichia coli Methods Enzymol. 2015;557:149-66. doi: 10.1016/bs.mie.2015.01.018. Epub 2015 Mar 24. PMID: 25950964 | ||||
Structure-based engineering of a minimal porin reveals loop-independent channel closure Biochemistry. 2014 Jul 29;53(29):4826-38. doi: 10.1021/bi500660q. Epub 2014 Jul 15. PMID: 24988371 | ||||
NMR-based conformational ensembles explain pH-gated opening and closing of OmpG channel J Am Chem Soc. 2013 Oct 9;135(40):15101-13. doi: 10.1021/ja408206e. Epub 2013 Oct 1. PMID: 24020969 | ||||
Mechanistic explanation of different unfolding behaviors observed for transmembrane and soluble ß-barrel proteins Structure. 2013 Aug 6;21(8):1317-24. doi: 10.1016/j.str.2013.06.001. Epub 2013 Jul 3. PMID: 23830738 | ||||
Structure of outer membrane protein G by solution NMR spectroscopy Proc Natl Acad Sci U S A. 2007 Oct 9;104(41):16140-5. doi: 10.1073/pnas.0705466104. Epub 2007 Oct 2. PMID: 17911261 | ||||
Structure of the monomeric outer-membrane porin OmpG in the open and closed conformation EMBO J. 2006 Aug 9;25(15):3702-13. doi: 10.1038/sj.emboj.7601237. Epub 2006 Aug 3. PMID: 16888630 | ||||
Crystal structure of the monomeric porin OmpG J Mol Biol. 2006 Jul 21;360(4):750-9. doi: 10.1016/j.jmb.2006.05.045. Epub 2006 Jun 2. PMID: 16797588 | ||||
Folding of a monomeric porin, OmpG, in detergent solution Biochemistry. 2003 Aug 12;42(31):9453-65. doi: 10.1021/bi0344228. PMID: 12899633 | ||||
Projection structure of the monomeric porin OmpG at 6 A resolution J Mol Biol. 2001 Jan 5;305(1):71-7. doi: 10.1006/jmbi.2000.4284. PMID: 11114248 | ||||
Biochemical and biophysical characterization of OmpG: A monomeric porin Biochemistry. 2000 Oct 3;39(39):11845-54. doi: 10.1021/bi001065h. PMID: 11009596 | ||||
| Proteins in this family with 3D-structure | ||||
View Entry | Description | Organism | Length | # strands |
