| Browse | Text search | Protein search | Domain search | Download | User manual | Related links | Citation & Contact |
The C-lobe and N-lobe beta barrels of Tf-binding protein B (TbpB_B_D) Family [Function: Non-specific diffusion channels] Seed alignment | Full alignment | Pfam page | ||||
Bacterial lipoproteins represent a large group of specialized membrane proteins that perform a variety of functions including maintenance and stabilization of the cell envelope, protein targeting and transit to the outer membrane, membrane biogenesis, and cell adherence. Pathogenic Gram-negative bacteria within the Neisseriaceae and Pasteurellaceae families rely on a specialized uptake system, characterized by an essential surface receptor complex that acquires iron from host transferrin (Tf) and transports the iron across the outer membrane. They have an iron uptake system composed of surface exposed lipoprotein, Tf-binding protein B (TbpB), and an integral outer-membrane protein, Tf-binding protein A (TbpA), that together function to extract iron from the host iron binding glycoprotein (Tf). TbpB is a bilobed (N and C lobe) lipid-anchored protein with each lobe consisting of an eight-stranded ß barrel flanked by four (N lobe) or eight (C lobe) ß strands. TbpB extends from the outer membrane surface by virtue of an N-terminal peptide region that is anchored to the outer membrane by fatty acyl chains on the N-terminal cysteine and is involved in the initial capture of iron-loaded Tf. This domain family is found in C and N lobe eight stranded ß barrel region of TbpB proteins. The eight-stranded barrel domains in N and C lobe draw comparisons to eight-stranded ß barrel outer-membrane protein W (OmpW). However, the barrel domains of TbpB have the hydrophobic residues line the inner surface of the ß barrels to create a stable hydrophobic core. | ||||
Representative image: 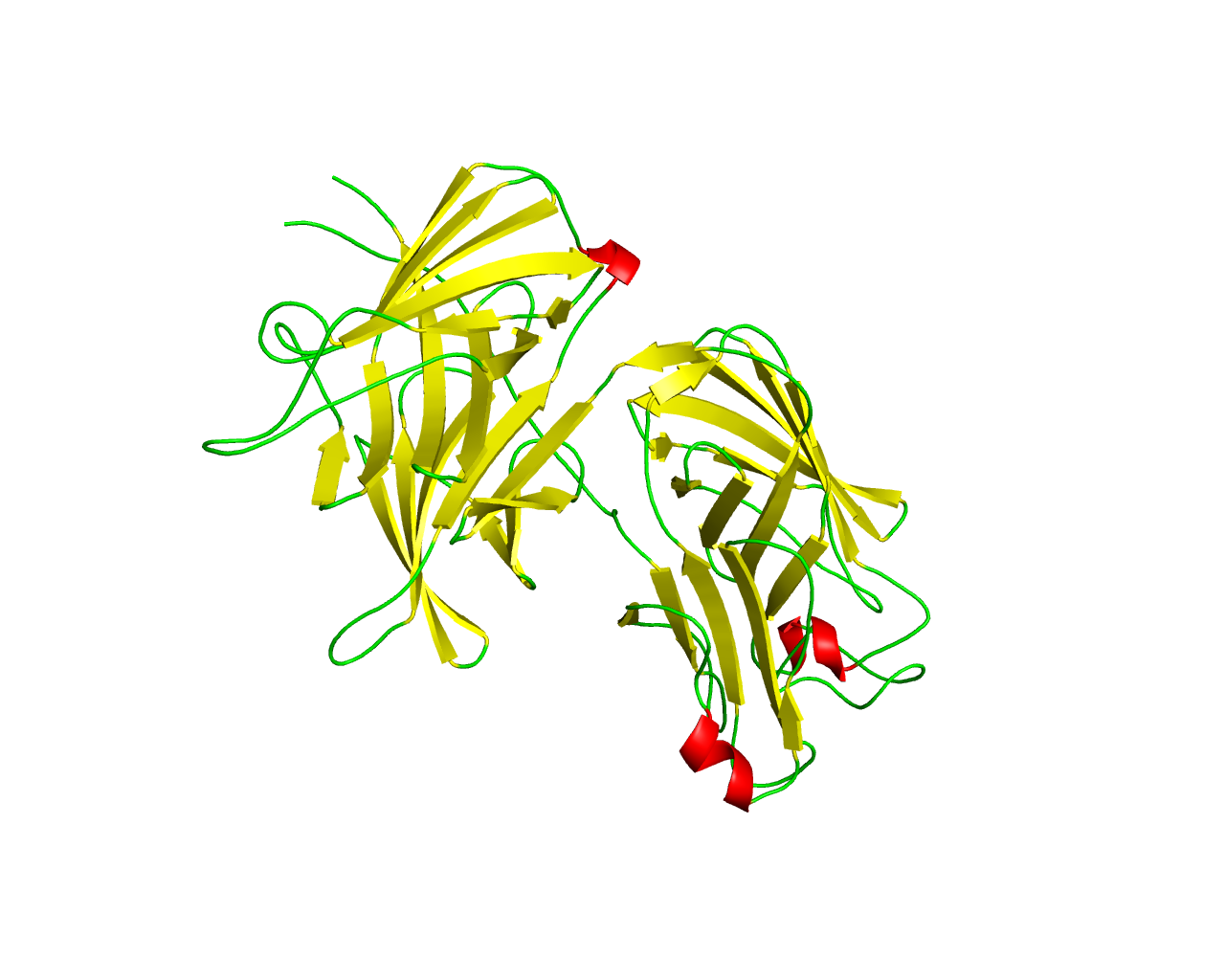 | ||||
| Literature references | ||||
Utility of Hybrid Transferrin Binding Protein Antigens for Protection Against Pathogenic Neisseria Species Front Immunol. 2019 Feb 19;10:247. doi: 10.3389/fimmu.2019.00247. eCollection 2019. PMID: 30837995 | ||||
Lactoferrin binding protein B - a bi-functional bacterial receptor protein PLoS Pathog. 2017 Mar 3;13(3):e1006244. doi: 10.1371/journal.ppat.1006244. eCollection 2017 Mar. PMID: 28257520 | ||||
Patterns of structural and sequence variation within isotype lineages of the Neisseria meningitidis transferrin receptor system Microbiologyopen. 2015 Jun;4(3):491-504. doi: 10.1002/mbo3.254. Epub 2015 Mar 19. PMID: 25800619 | ||||
Pyrophosphate-mediated iron acquisition from transferrin in Neisseria meningitidis does not require TonB activity PLoS One. 2014 Oct 7;9(10):e107612. doi: 10.1371/journal.pone.0107612. eCollection 2014. PMID: 25290693 | ||||
Characterization of Mannheimia haemolytica isolated from feedlot cattle that were healthy or treated for bovine respiratory disease Can J Vet Res. 2014 Jan;78(1):38-45. PMID: 24396179 | ||||
Conserved regions of gonococcal TbpB are critical for surface exposure and transferrin iron utilization Infect Immun. 2013 Sep;81(9):3442-50. doi: 10.1128/IAI.00280-13. Epub 2013 Jul 8. PMID: 23836816 | ||||
Structural insight into the lactoferrin receptors from pathogenic Neisseria J Struct Biol. 2013 Oct;184(1):83-92. doi: 10.1016/j.jsb.2013.02.009. Epub 2013 Feb 24. PMID: 23462098 | ||||
Steric and allosteric factors prevent simultaneous binding of transferrin-binding proteins A and B to transferrin Biochem J. 2012 Jun 1;444(2):189-97. doi: 10.1042/BJ20112133. PMID: 22369045 | ||||
Crystal structure of the N-lobe of lactoferrin binding protein B from Moraxella bovis Biochem Cell Biol. 2012 Jun;90(3):351-61. doi: 10.1139/o11-078. Epub 2012 Feb 14. PMID: 22332934 | ||||
Structural basis for iron piracy by pathogenic Neisseria Nature. 2012 Feb 12;483(7387):53-8. doi: 10.1038/nature10823. PMID: 22327295 | ||||
Insights into the bacterial transferrin receptor: the structure of transferrin-binding protein B from Actinobacillus pleuropneumoniae Mol Cell. 2009 Aug 28;35(4):523-33. doi: 10.1016/j.molcel.2009.06.029. PMID: 19716795 | ||||
The solution structure of the outer membrane lipoprotein OmlA from Xanthomonas axonopodis pv. citri reveals a protein fold implicated in protein-protein interaction Proteins. 2008 Jun;71(4):2051-64. doi: 10.1002/prot.21886. PMID: 18186471 | ||||

|

|

|

|
