| Browse | Text search | Protein search | Domain search | Download | User manual | Related links | Citation & Contact |
The C-lobe handle domain of Tf-binding protein B (TbpB_C) Family [Function: Active transporters] Seed alignment | Full alignment | Pfam page | ||||
Bacterial lipoproteins form a large group of specialized membrane proteins that are involved in a variety of functions including maintenance and stabilization of the cell envelope, protein targeting and transit to the outer membrane, membrane biogenesis, and cell adherence. Pathogenic Gram-negative bacteria within the Neisseriaceae and Pasteurellaceae families rely on a specialized uptake system, characterized by an essential surface receptor complex that acquires iron from host transferrin (Tf) and transports the iron across the outer membrane. They have an iron uptake system composed of surface exposed lipoprotein, Tf-binding protein B (TbpB), and an integral outer-membrane protein, Tf-binding protein A (TbpA), that together function to extract iron from the host iron binding glycoprotein (Tf). TbpB is a bilobed (N and C lobe) lipid-anchored protein with each lobe consisting of an eight-stranded ß barrel flanked by a handle domain made up of four (N lobe) or eight (C lobe) ß strands. TbpB extends from the outer membrane surface by virtue of an N-terminal peptide region that is anchored to the outer membrane by fatty acyl chains on the N-terminal cysteine and is involved in the initial capture of iron-loaded Tf. This domain family is found in the handle domain of the C lobe (domain C) of TbpB proteins. It consists of a squashed six-stranded ß sheet flanked by two antiparallel ß strands and has no supporting alpha helix as in the N lobe. | ||||
Representative image: 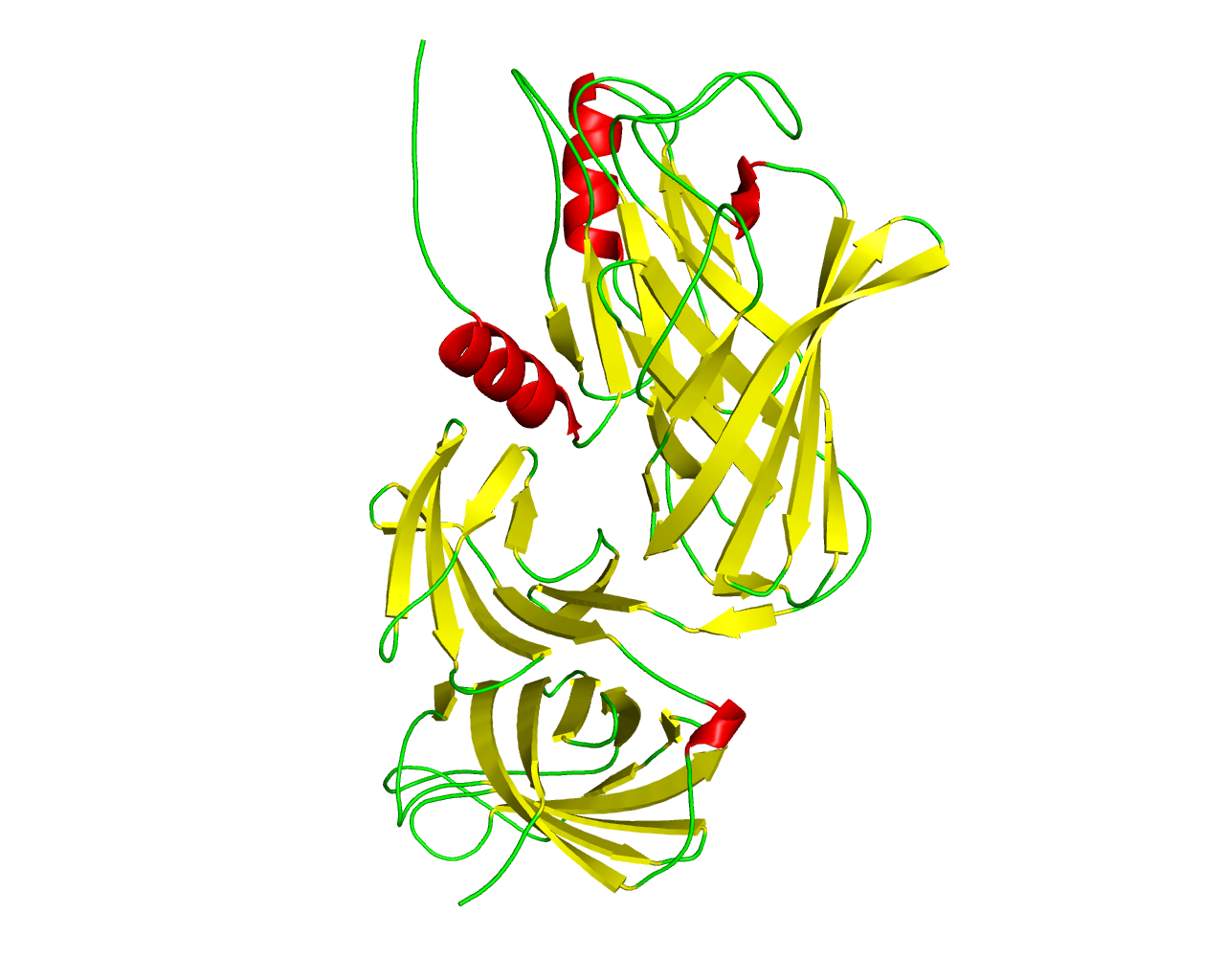 | ||||
| Literature references | ||||
Identification of a Large Family of Slam-Dependent Surface Lipoproteins in Gram-Negative Bacteria Front Cell Infect Microbiol. 2017 May 31;7:207. doi: 10.3389/fcimb.2017.00207. eCollection 2017. PMID: 28620585 | ||||
Lactoferrin binding protein B - a bi-functional bacterial receptor protein PLoS Pathog. 2017 Mar 3;13(3):e1006244. doi: 10.1371/journal.ppat.1006244. eCollection 2017 Mar. PMID: 28257520 | ||||
Iron acquisition through the bacterial transferrin receptor Crit Rev Biochem Mol Biol. 2017 Jun;52(3):314-326. doi: 10.1080/10409238.2017.1293606. Epub 2017 Mar 1. PMID: 28276700 | ||||
Patterns of structural and sequence variation within isotype lineages of the Neisseria meningitidis transferrin receptor system Microbiologyopen. 2015 Jun;4(3):491-504. doi: 10.1002/mbo3.254. Epub 2015 Mar 19. PMID: 25800619 | ||||
Conserved regions of gonococcal TbpB are critical for surface exposure and transferrin iron utilization Infect Immun. 2013 Sep;81(9):3442-50. doi: 10.1128/IAI.00280-13. Epub 2013 Jul 8. PMID: 23836816 | ||||
Structural variations within the transferrin binding site on transferrin-binding protein B, TbpB J Biol Chem. 2011 Apr 8;286(14):12683-92. doi: 10.1074/jbc.M110.206102. Epub 2011 Feb 5. PMID: 21297163 | ||||
Insights into the bacterial transferrin receptor: the structure of transferrin-binding protein B from Actinobacillus pleuropneumoniae Mol Cell. 2009 Aug 28;35(4):523-33. doi: 10.1016/j.molcel.2009.06.029. PMID: 19716795 | ||||
The solution structure of the outer membrane lipoprotein OmlA from Xanthomonas axonopodis pv. citri reveals a protein fold implicated in protein-protein interaction Proteins. 2008 Jun;71(4):2051-64. doi: 10.1002/prot.21886. PMID: 18186471 | ||||

|

|

|

|
