| Browse | Text search | Protein search | Domain search | Download | User manual | Related links | Citation & Contact |
The OmpA-like transmembrane domain Family [Function: Structural] Seed alignment | Full alignment | Pfam page | Pfam Wiki page | TC-DB page | ||||
This family includes homologs of the well characterized OmpA protein of Escherichia coli. Members of this family (similar to members of the OprF family) show a modular architecture which is composed of two distinctstructural domains: an N-terminal ß-barrel domain formed by 8 ß strands with short turns at the periplasmic ends and long flexible loops at the external ends that anchors the protein to the outer membrane; and a C-terminal domain that protrudes into the periplasmic space interacting with peptidoglycans. The C-terminal domain (named OmpA-like domain), is also found in a number of outer membrane lipoproteins, therefore it is not characteristic of ß-barrels. OmpA is largely expressed in the outer membrane of Escherichia coli and is required for bacterial conjugation and for maintenance of outer membrane stability. OmpA of E. coli K1 is a potential vaccine candidate because of its predominant contribution to bacterial pathogenesis and sub-cellular localization.There is also evidence that OmpA exhibits a small channel activity. There is a distant homology to Neisserial opacity (Opa) adhesins and members of the Ail/Lom/OmpX family but the barrels, though they possess the same number of strands (8), show different characteristics (shear numbers, inclination with respect to the membrane). A molecular-dynamics simulations of the full-length model of the OmpA dimer proposed by Robinson and co-workers revealed a large dimerization interface within the membrane environment, ensuring that the dimer is stable over the course of the simulations. The linker is flexible, expanding and contracting to pull the globular C-terminal domain up toward the membrane or push it down toward the periplasm, suggesting a possible mechanism for providing mechanical stability to the cell. | ||||
Representative image: 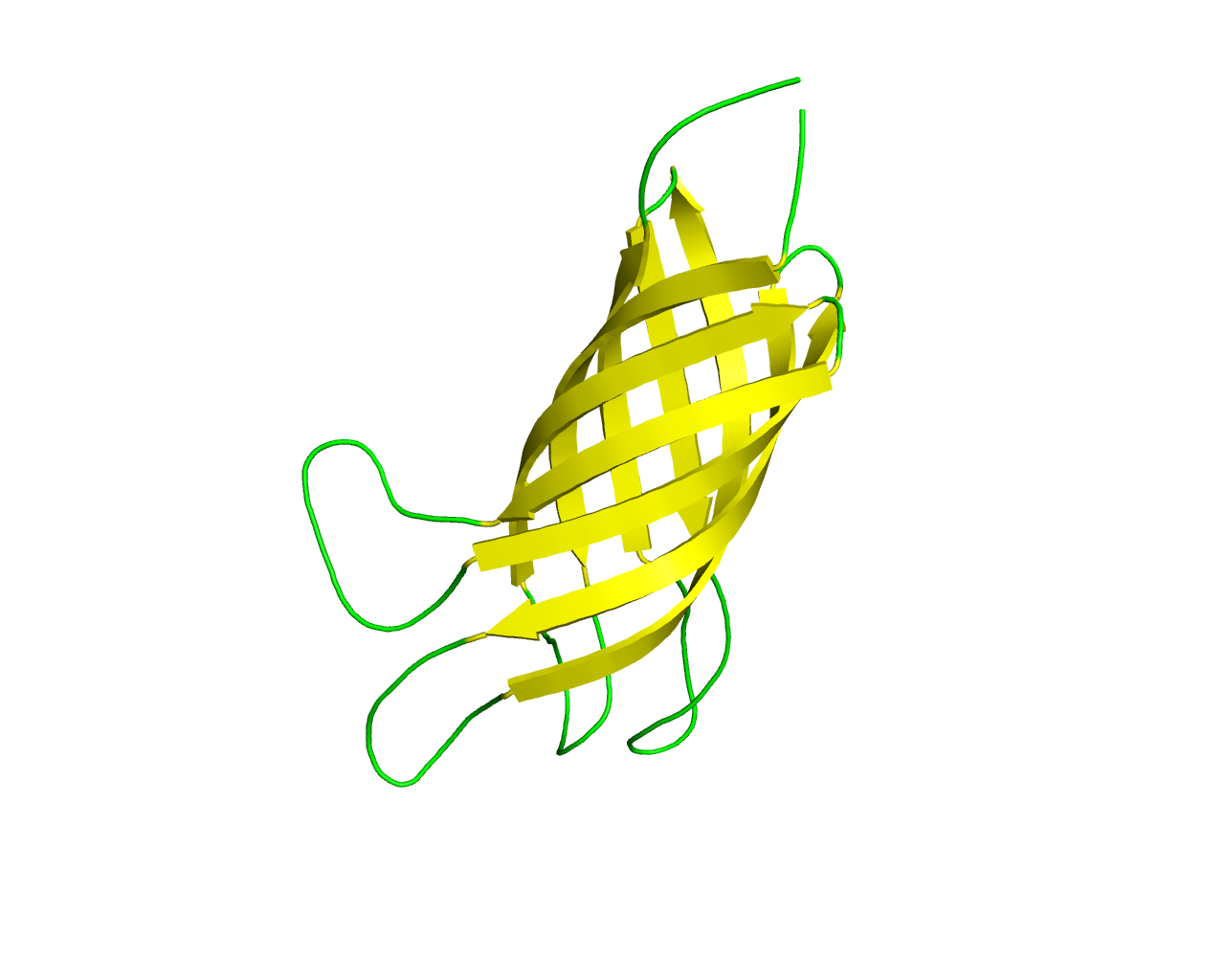 | ||||
| Literature references | ||||
Microevolution in the major outer membrane protein OmpA of Acinetobacter baumannii Microb Genom. 2020 Jun;6(6):e000381. doi: 10.1099/mgen.0.000381. Epub 2020 Jun 4. PMID: 32496178 | ||||
Magnetite-OmpA Nanobioconjugates as Cell-Penetrating Vehicles with Endosomal Escape Abilities ACS Biomater Sci Eng. 2020 Jan 13;6(1):415-424. doi: 10.1021/acsbiomaterials.9b01214. Epub 2019 Dec 2. PMID: 33463215 | ||||
Folding of the ß-Barrel Membrane Protein OmpA into Nanodiscs Biophys J. 2020 Jan 21;118(2):403-414. doi: 10.1016/j.bpj.2019.11.3381. Epub 2019 Nov 28. PMID: 31843264 | ||||
Outer Membrane Proteins OmpA, FhuA, OmpF, EstA, BtuB, and OmpX Have Unique Lipopolysaccharide Fingerprints J Chem Theory Comput. 2019 Apr 9;15(4):2608-2619. doi: 10.1021/acs.jctc.8b01059. Epub 2019 Mar 21. PMID: 30848905 | ||||
Rational Design and Evaluation of an Artificial Escherichia coli K1 Protein Vaccine Candidate Based on the Structure of OmpA Front Cell Infect Microbiol. 2018 May 23;8:172. doi: 10.3389/fcimb.2018.00172. eCollection 2018. PMID: 29876324 | ||||
The Bam complex catalyzes efficient insertion of bacterial outer membrane proteins into membrane vesicles of variable lipid composition J Biol Chem. 2018 Feb 23;293(8):2959-2973. doi: 10.1074/jbc.RA117.000349. Epub 2018 Jan 8. PMID: 29311257 | ||||
Insights into PG-binding, conformational change, and dimerization of the OmpA C-terminal domains from Salmonella enterica serovar Typhimurium and Borrelia burgdorferi Protein Sci. 2017 Sep;26(9):1738-1748. doi: 10.1002/pro.3209. Epub 2017 Jun 19. PMID: 28580643 | ||||
Local and Global Dynamics in Klebsiella pneumoniae Outer Membrane Protein a in Lipid Bilayers Probed at Atomic Resolution J Am Chem Soc. 2017 Feb 1;139(4):1590-1597. doi: 10.1021/jacs.6b11565. Epub 2017 Jan 20. PMID: 28059506 | ||||
Novel Kinetic Intermediates Populated along the Folding Pathway of the Transmembrane ß-Barrel OmpA Biochemistry. 2017 Jan 10;56(1):47-60. doi: 10.1021/acs.biochem.6b00809. Epub 2016 Dec 21. PMID: 28001375 | ||||
Concatemers of Outer Membrane Protein A Take Detours in the Folding Landscape Biochemistry. 2016 Dec 27;55(51):7123-7140. doi: 10.1021/acs.biochem.6b01153. Epub 2016 Dec 14. PMID: 27973779 | ||||
Full-Length OmpA: Structure, Function, and Membrane Interactions Predicted by Molecular Dynamics Simulations Biophys J. 2016 Oct 18;111(8):1692-1702. doi: 10.1016/j.bpj.2016.09.009. PMID: 27760356 | ||||
Aqueous, Unfolded OmpA Forms Amyloid-Like Fibrils upon Self-Association PLoS One. 2015 Jul 21;10(7):e0132301. doi: 10.1371/journal.pone.0132301. eCollection 2015. PMID: 26196893 | ||||
The Borrelia afzelii outer membrane protein BAPKO_0422 binds human factor-H and is predicted to form a membrane-spanning ß-barrel Biosci Rep. 2015 Jul 9;35(4):e00240. doi: 10.1042/BSR20150095. PMID: 26181365 | ||||
Detecting envelope stress by monitoring ß-barrel assembly Cell. 2014 Dec 18;159(7):1652-64. doi: 10.1016/j.cell.2014.11.045. PMID: 25525882 | ||||
Recombinant outer membrane protein A fragments protect against Escherichia coli meningitis J Microbiol Immunol Infect. 2016 Jun;49(3):329-34. doi: 10.1016/j.jmii.2014.07.012. Epub 2014 Oct 8. PMID: 25305709 | ||||
Solution state NMR structure and dynamics of KpOmpA, a 210 residue transmembrane domain possessing a high potential for immunological applications J Mol Biol. 2009 Jan 9;385(1):117-30. doi: 10.1016/j.jmb.2008.10.021. Epub 2008 Oct 15. PMID: 18952100 | ||||
A minimal transmembrane beta-barrel platform protein studied by nuclear magnetic resonance Biochemistry. 2007 Feb 6;46(5):1128-40. doi: 10.1021/bi061265e. PMID: 17260943 | ||||
Increasing the accuracy of solution NMR structures of membrane proteins by application of residual dipolar couplings. High-resolution structure of outer membrane protein A J Am Chem Soc. 2006 May 31;128(21):6947-51. doi: 10.1021/ja0608343. PMID: 16719475 | ||||
Structure of the OmpA-like domain of RmpM from Neisseria meningitidis Mol Microbiol. 2004 Feb;51(4):1027-37. doi: 10.1111/j.1365-2958.2003.03903.x. PMID: 14763978 | ||||
OmpA membrane domain as a tight-binding anchor for lipid bilayers Chembiochem. 2002 May 3;3(5):463-6. doi: 10.1002/1439-7633(20020503)3:5<463::AID-CBIC463>3.0.CO;2-P. PMID: 12007182 | ||||
Structure of outer membrane protein A transmembrane domain by NMR spectroscopy Nat Struct Biol. 2001 Apr;8(4):334-8. doi: 10.1038/86214. PMID: 11276254 | ||||
High-resolution structure of the OmpA membrane domain J Mol Biol. 2000 Apr 28;298(2):273-82. doi: 10.1006/jmbi.2000.3671. PMID: 10764596 | ||||
Structure of the outer membrane protein A transmembrane domain Nat Struct Biol. 1998 Nov;5(11):1013-7. doi: 10.1038/2983. PMID: 9808047 | ||||
| Proteins in this family with 3D-structure | ||||
View Entry | Description | Organism | Length | # strands |
